Describe the Method Used to Identify Carbon Dioxide Gas
A lighted splint can be used as a preliminary test for carbon dioxide. Carbon dioxide dissolves in water to form carbonic acid H 2 CO 3.
Generating Collecting And Testing Gases Experiment Rsc Education
When the tap is closed the carbon dioxide forces the acid back down to the.
. Carbon sequestration is the process of capturing and storing atmospheric carbon dioxide. It is one method of reducing the amount of carbon dioxide in the atmosphere with the goal of reducing global climate change. There are several tests for carbon dioxide that can be conducted in different settings.
Tighten the pump to tightly hold the tube if your pump has this. Carbon dioxide produces a white precipitate in limewater. Write the formula for calculating the cylinder factor for a gas-filled cylinder.
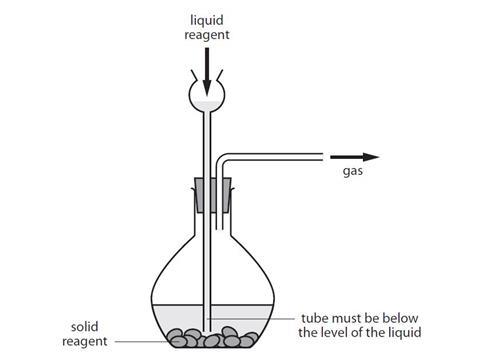
After capture carbon dioxide CO 2 is compressed and then transported to a site where it is injected underground for permanent storage also known as sequestration. Double-check that the acid is hydrochloric and NOT sulfuric. Carbon dioxide gas extinguishes the lighted splint as iot does not supports combustion.
Liquid-filledThe most accurate way to determine the contents of a liquid-filled cylinder is to weigh the cylinder. Refer to CLEAPSS Hazcards HC022a HC047a and HC081 plus CLEAPSS Recipe Book RB024. Pass the gas into limewater.
CO 2 is commonly transported by pipeline but it can also be transported by train truck or ship. Carbon dioxide is also exchanged between the atmosphere and the oceans. In 1772 English chemist Joseph Priestley published a paper entitled Impregnating Water with Fixed Air in which he described a process of dripping sulfuric acid or oil of vitriol as Priestley knew it on chalk in order to produce carbon dioxide and forcing the gas to dissolve by agitating a bowl of water in contact with the gas.
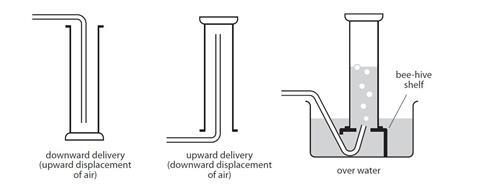
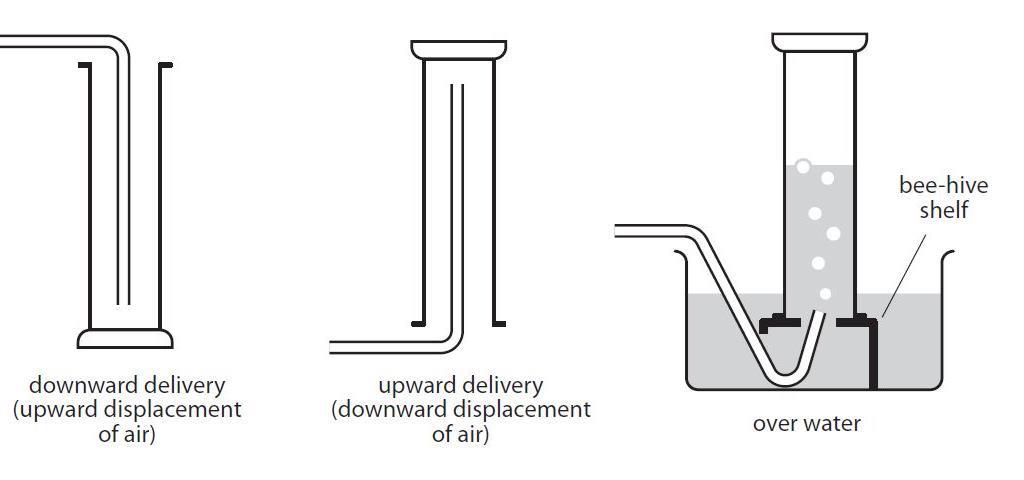
People and animals inhale oxygen from the air and exhale carbon dioxide CO 2 while plants absorb CO 2 for photosynthesis and emit oxygen back into the atmosphere. Methods for collecting gases Downward delivery for gases that are denser than air. 1 Carbon dioxide gas CO 2 g has no colour or smell.
Add 14 cm 3 of concentrated hydrochloric acid CORROSIVE to at least 3 g of potassium manganate VII OXIDISING HARMFUL and DANGEROUS FOR THE ENVIRONMENT. Starting in 1958 Charles Keeling used the scientific method to take meticulous measurements of atmospheric carbon dioxide CO 2 at Mauna Loa Observatory in Waimea Hawaii. Cylinder factor Cubic feet full 283.
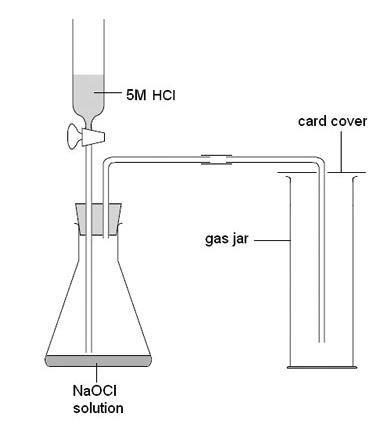
Collection in gas syringe when the volume of gas needs to be measured. The gas liberated in the chemical reaction is passed via a delivery tube into a solution of limewaterchemically calcium hydroxide. Descriptions like cloudy or milky are not really correct.
The figure below depicts the carbon. Purified air is liquefied by compression and cooled by rapid expansion. What are the General Tests for Carbon Dioxide Gas.
2 Carbon dioxide gas will put out a lit splint. Once the pressure temperature and volume of a gas are known the amount of gas can be calculated taking the temperature dependent compressibility of the gases into account. Damp litmus paper held in a test tube.
4 rows Place a burning splint into a test tube and if it goes out it could be carbon dioxide. Pressure full in psig. The best way of testing for Carbon dioxide is to bubble it through lime water.
This graph known as the Keeling Curve shows how atmospheric CO 2 has continued rising since then. Limewater is basically a solution of calcium hydroxide. Carbon dioxide was first liquefied at elevated.
Pull pump handle and wait 2 minutes or until vacuum. Limewater solution turning milkycloudy indicates that the gas liberated was CO2. Most therapeutic gases will oxidize or support combustion.
Carbon dioxide is the most commonly produced greenhouse gas. Describe the four basic steps of the fractional distillation process. Lime water turns milky as the Calcium hydroxide chemical name for limewater reacts with carbon dioxide to form Calcium Carbonate which is insoluble in water and thus forms a milky white precipitate.
One of the most common tests for carbon dioxide gasCO2 is the limewater test. List three gases in this category. So if we bubble carbon dioxide through the solution it reacts with calcium hydroxide solution to produce a white precipitate of.
Hydrogen Upward delivery for gases that are less dense than air. When the tap is opened the carbon dioxide fl ows out reducing the gas pressure in the middle chamber. 844b Describe and use the test to identify carbon dioxide gas.
Then the air with the CO 2 that is to be determined still in it is slowly and completely flowed at low pressure over a cold trap cooled with liquid nitrogen. This allows acid to rise from the bottom chamber and react with the marble chips. Confirmatory Test for Carbon Dioxide.
What is the Test for Carbon Dioxide. Set the pump to a 100 mL stroke most pumps can do either 50 mL or 100 mL strokes Break both ends of the carbon dioxide test tube off. 2 Identify the properties of hydrogen ammonia and carbon dioxide gases 1 Use of from IT CIS5302 at University of Southern Queensland.
The most common and easy ones are as follows. Geologic formations suitable for sequestration include depleted oil and gas fields deep coal seams and. 844b Describe and use the test to identify carbon dioxide gas.
3 Carbon dioxide gas will turn moist litmus paper from blue to red and moist universal indicator paper to yellow. A positive test will result in the lime water turning milky. 844b Describe and use the test to identify carbon dioxide gas.
Atmospheric air is filtered to remove pollutants water and carbon dioxide. Insert CO2 test tube into the hand pump with the air flow arrow pointing towards the pump. Carbonic acid is a weak acid see the.
The USGS is conducting assessments on two major types of carbon sequestration. Up to 24 cash back The Kipps apparatus uses this reaction to produce carbon dioxide. Air oxygen and nitric oxide.
Carbon dioxide is present. Eg carbon dioxide Collection over water for gases that are not very soluble in water. Gas bubbled through limewater.
Limewater turns milky or cloudy white. This natural system of processes keeps CO 2 levels in the atmosphere stable over time.
Generating Collecting And Testing Gases Experiment Rsc Education

Generating Collecting And Testing Gases Experiment Rsc Education
Generating Collecting And Testing Gases Experiment Rsc Education

Limewater Test To Check For Carbon Dioxide In Your Breath Videos Examples Activities
No comments for "Describe the Method Used to Identify Carbon Dioxide Gas"
Post a Comment